PregnanSEE: A Point-of-Care Blood Test for Pregnant Women in Rural India
Our group wanted to focus on women’s health issues and also liked the idea of international and rural applications. This led us to research issues involving pregnancy in rural India. We identified four common deficiencies in pregnant women in rural India that had related health issues. The biomarkers we chose that commonly had associated deficiencies in pregnant women in rural India were vitamin B9, vitamin B12, ferritin, and hemoglobin. Ferritin and hemoglobin were chosen as they are both measured in a full blood iron panel and are a good indication of iron levels in the body. Our group decided that designing a minimally invasive and inexpensive diagnostic device was our desired course of action. My team consisted of fellow master’s students Preethika Chivukula, Albert Fernández, Ragini Kothari, Shiv Patil, Delfina Rodriguez, and myself. After the first semester, Shiv and Preethika stepped back allowing Ava Heller and Pragya Gupta to join the team. This project was done as part of our Biomedical Innovation class at Columbia University. Our team was presented with the award for best innovation in our Biomedical Innovation class at the end of the first semester by a group of judges that consisted of medical professionals and Columbia University faculty. This project began in September 2021 and continued through May 2022.
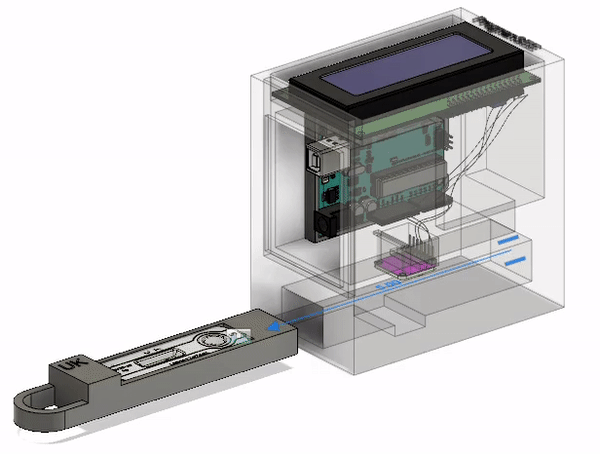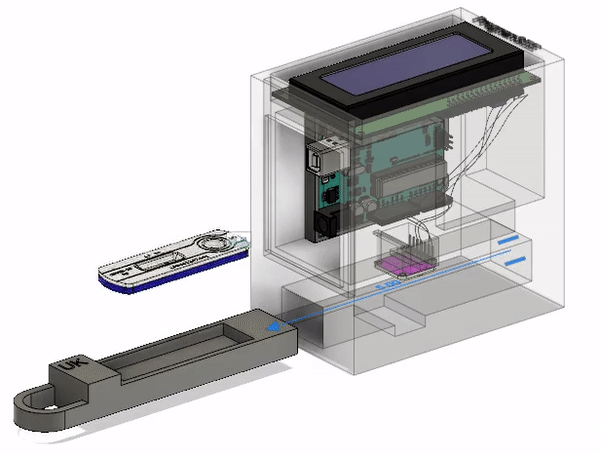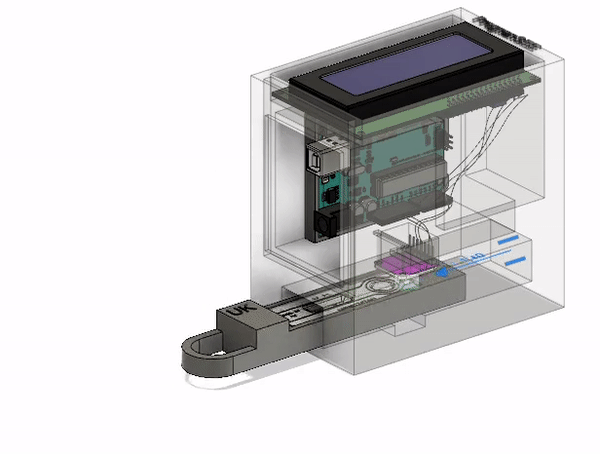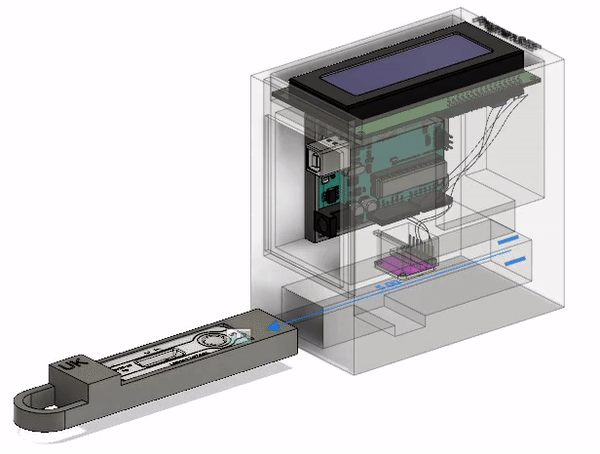
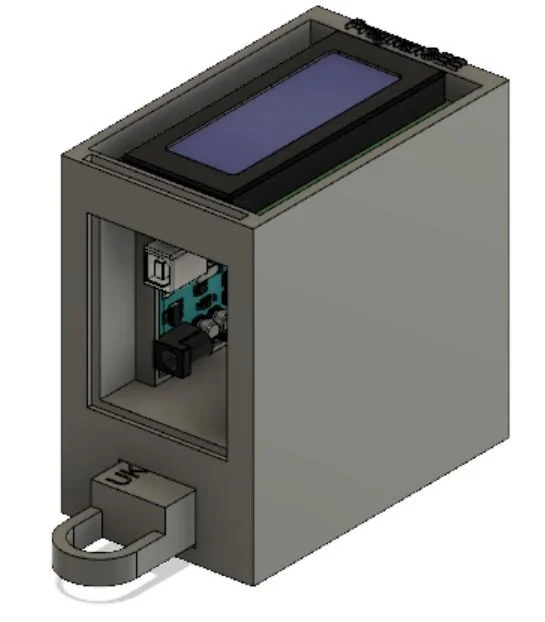
Final Prototype
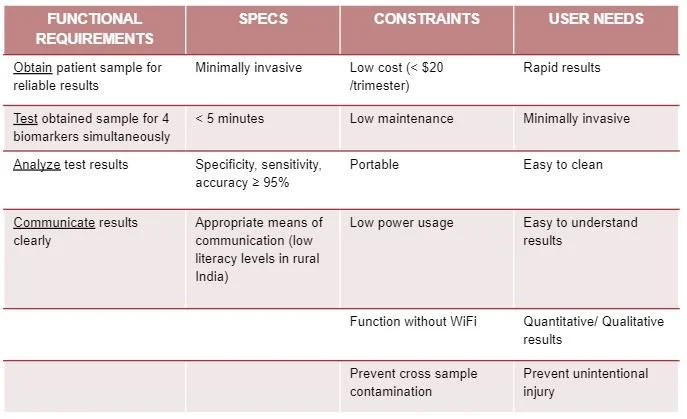
This is the most current model of our prototype. It consists of an RGB sensor, an Arduino Uno, and an LCD screen along with 3D printed parts. The LFA test strip is first placed in the drawer. The drawer is then placed into the device and the LFA test is given time to complete. At that point, the results will be read and displayed on the screen for the ASHA worker to interpret and communicate to the patient. The CAD model that I designed below illustrates this action. The mechanisms involved will be discussed further below.
Background
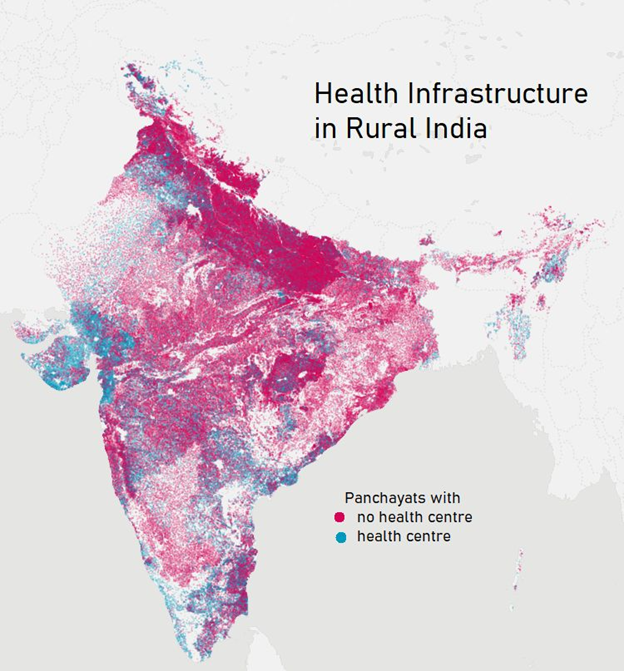
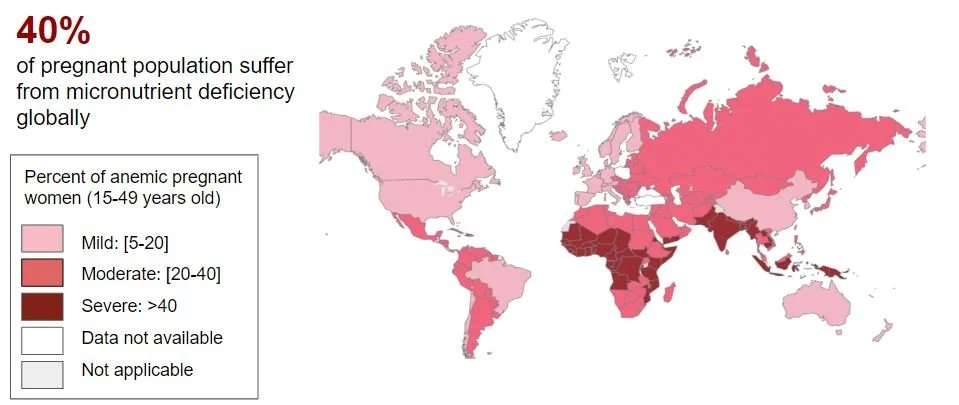
The healthcare infrastructure in rural India is not very accessible as we can see displayed on the map. 73% of villages do not have access to a health center with doctors, nurses, and lab techs. And about 90 million people have to travel more than 10 km to reach a health center. These are some reasons we thought a point-of-care device would be most helpful to these communities.
“No title,” Twitter.com. [Online]. Available: https://mobile.twitter.com/jeevika_shiv/status/1391709519625981954. [Accessed: 14-Dec-2021].
More Background- ASHA Workers
ASHA workers are accredited social health activists. ASHA workers are women who live in these villages and are trained in performing basic health check-ups and disseminating health information. They move door to door keeping track of the community’s health and connecting them with the public health system. Lastly, they assign each pregnant patient an antenatal card where the patient's vitals, supplementation, and future check-ups are recorded and tracked. We envision the ASHA workers to be the ones who will use our device and interpret the results for the pregnant women. ASHA workers already carry and provide supplementation for deficiencies in these four biomarkers but there is a large lack of motivation on the part of the pregnant mothers to take the supplements which is one thing we hope our device will help with.
“Empowering the ASHA worker,” Wadhwani AI, 2020. [Online]. Available: https://www.wadhwaniai.org/2020/11/empowering-the-asha-worker/.
Deficiency Prevalence and Associated Health Problems
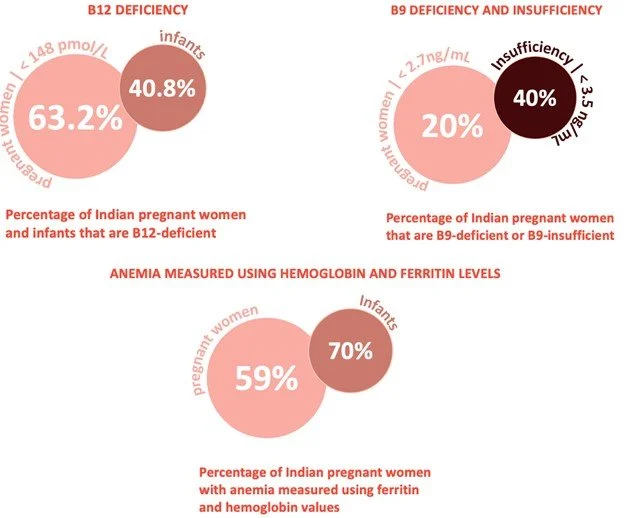
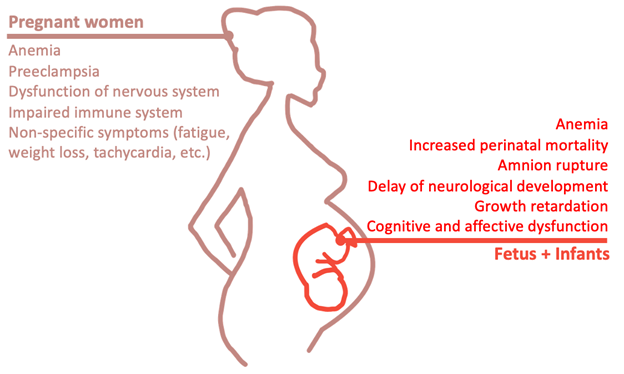
In the graphic on the left created by one of my team members, you can see the prevalence of deficiencies in some of our chosen biomarkers between pregnant women and infants in India. On the right, you can see the common negative health effects of these deficiencies for both the pregnant mother and their child. As you can see the biggest overarching health issue for both the mother and the child due to these deficiencies is anemia. The graphic on the right was also created by one of our team members.
B12: J. L. Finkelstein, A. Fothergill, J. T. Krisher, T. Thomas, A. V. Kurpad, and P. Dwarkanath, “Maternal vitamin B12 deficiency and perinatal outcomes in southern India,” PLoS One, vol. 16, no. 4, p. e0248145, Apr. 2021, doi: 10.1371/journal.pone.0248145.
B9: Bhide, P., Kar, A. Prevalence and determinants of folate deficiency among urban Indian women in the periconception period. Eur J Clin Nutr 73, 1639–1641 (2019). https://doi.org/10.1038/s41430-018-0255-2
Anemia: Finkelstein, J.L., Kurpad, A.V., Bose, B. et al. Anaemia and iron deficiency in pregnancy and adverse perinatal outcomes in Southern India. Eur J Clin Nutr74, 112–125 (2020). https://doi.org/10.1038/s41430-019-0464-3
Interviews and Stakeholder Validation
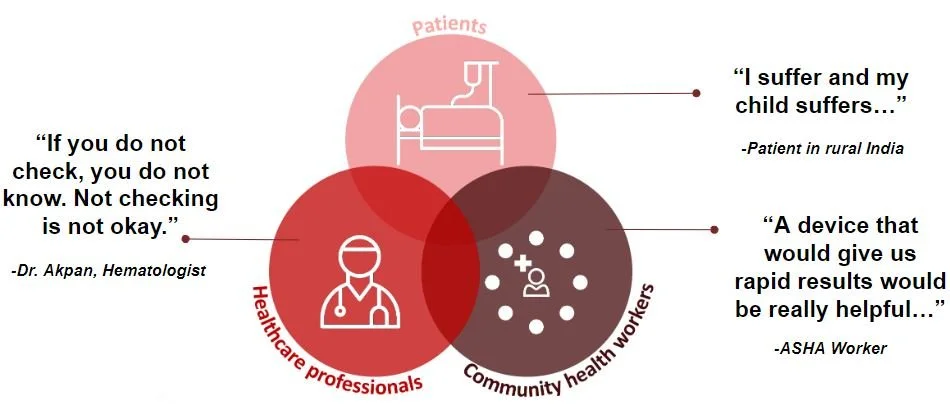
We interviewed many people including a variety of stakeholders spanning from patients to healthcare professionals and community health workers like the aforementioned ASHA workers. Some key insights that truly validated our need really occurred after interviewing and speaking to an ASHA worker where it was explicitly stated that a device that would give rapid results would be extremely helpful especially since there aren’t really any reliable means of transportation to hospitals in rural India. She also validated that proper levels of these four specific biomarkers are crucial for fetal development. Additionally, Dr. Akpan, who runs a women’s health clinic for several blood disorders, validated our unmet need by stating that there truly is merit in proper diagnosis of these risk factors because it serves as motivation for mothers to take their supplements or take action on their specific course of treatment.
Design Inputs
Here you can see our design inputs. The important ones I want to highlight are that the device is low cost, making it accessible to ASHA workers within their given budget, functions without Wi-Fi, so that it can be used in rural communities, and safe, so that our device does not cause any cross contamination or health issues.
Prototype Functionality
Our device will use a lateral flow immunoassay to test the sample. First, the patient will have their finger pricked by the ASHA worker using a lancet, which the ASHA workers are already trained to do and currently do. Next, the patient will put a drop of blood, followed by a few drops of buffer solution, onto our test strip which will be sitting in a drawer. Then, the drawer with the test strip inside will be placed into our device and the test will be completed. Following that, the results of the test will be quantified using a color sensor, the RGB sensor mentioned above. With the quantified results, the levels of the biomarkers will be displayed on the screen and then interpreted by the ASHA workers and relayed to the patient. Lateral flow immunoassays generally give binary results where the test line is either present or not present such as in a pregnancy test. However, according to our research, we believe we can quantify the test results by measuring the darkness of the test line in order to generate a corresponding concentration in the patient’s blood. We would need to first calibrate our device using solutions of known concentrations of our biomarkers in order to properly have our device determine the corresponding concentrations from the darkness of the test lines. We also believe we can test for all four of our biomarkers in one test strip but need to confirm this with further testing.
Initial Prototype Model
This is the initial model of our prototype that I designed in Fusion 360. The idea was that the patient would prick their finger on the spike on the bottom of the device, then input their finger into the hole in the bottom to put their drop of blood onto our test strip. the two buttons on the front are used to operate the device and the square indent is where the screen would be to display the results. The dimensions of this model are 100mm x 50mm x 50mm. After much discussion, the team determined that pricking the finger on the device itself would lead to possible cross-contamination and would not be viable safety-wise for our device. This lead us to decide that the patient’s finger would be pricked with a separate lancet that we would provide by the ASHA worker. We also determined that having the patient insert their whole finger with an open wound into the device would cause further possibility for contamination and decided that the blood should be placed onto the test strip outside of the device.
Second Prototype Model
This is the final model of our prototype that has improvements from the first model determined by team discussion and research. This model was also designed by myself in Fusion 360. This version of the prototype model has a drawer that houses the test strip rather than a hole in the bottom of the device for the patient to input their finger into. We decided that this would significantly decrease the possibility of cleanliness issues. The corners of the device were also rounded out to make it safer and more visually appealing. The part of the model where the patient pricks their finger has also been removed in this model as it was determined the finger of the patient would be pricked by the ASHA worker with a separate lancet. The drawer would be cleaned by the ASHA worker after each use to ensure safety and prevent cross-contamination.
Initial Proof of Concept
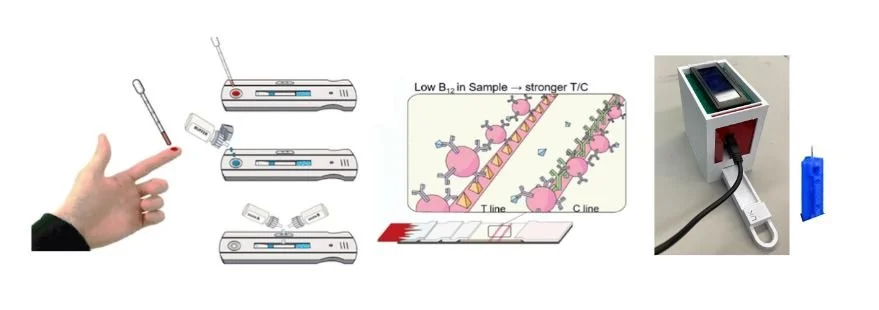
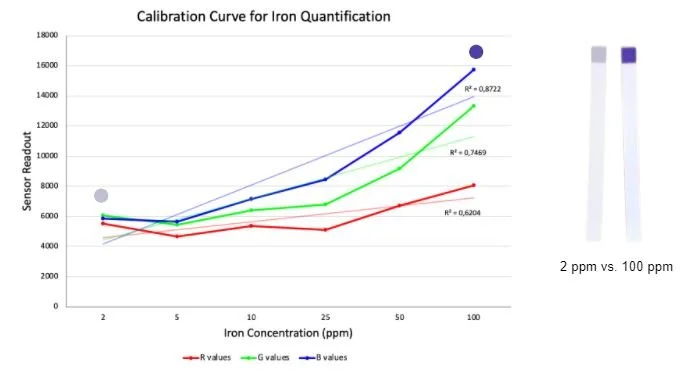
Due to time constraints we were unable to perform testing with an actual lateral flow immunoassay and our chosen biomarkers. Instead, we used solutions with different iron concentrations and commercially available iron test strips. The concept we wanted to prove was that we could use an RGB sensor to quantify the concentration of a substance in a solution based on the darkness of the test result. The iron tests we used gave qualitative results using different shades of blue to correspond to different concentrations of iron. We created solutions with different known concentrations and used the test strip on each solution. We then quantified the darkness of each strip using an RGB sensor connected to an Arduino. We used the solutions with known concentrations and corresponding RGB values to construct a calibration curve that would allow us to determine the concentration in an unknown solution using the RGB values, thus proving our concept. As you can see from the graph on the right the results were quite promising. As the test results were given in shades of blue, we would expect the trendline for the blue values given from the RGB sensor to be the closest to what we wanted which is what we see in the graph. While the results were not perfect they were certainly promising and warrant further testing. For our device in the future, we would need to create these calibration curves for all four of our biomarkers. We would do this by creating solutions with known concentrations of each one, then running the solutions through our lateral flow immunoassay and quantifying the darkness of the resultant test lines using the RGB sensor and Arduino.
Images of products from Amazon
Third Prototype
This was the first working prototype that we developed. This is also the prototype used in our killer experiment. I designed this prototype in CAD with input from my teammates. This prototype was a bit bulkier than we were looking for as our device is meant to be a tool carried by ASHA workers on their person for day-to-day use. The bulkiness led us to the next prototype model. This prototype included the RGB sensor, an Arduino, and an LCD screen. The rest of the parts were 3D printed in the Columbia Biomedical Engineering Lab.
Fourth and Most Recent Prototype
This is the most recent prototype. It is very similar to our third prototype but we cut the size down a lot to a much more condensed version that would be easier for the ASHA workers to carry. This prototype was also functional but we had started experimentation with the third prototype and decided to continue with that one to keep the experimentation consistent. This prototype was another that I designed in CAD with the final CAD model being seen in the image on the left.
Killer Experiment
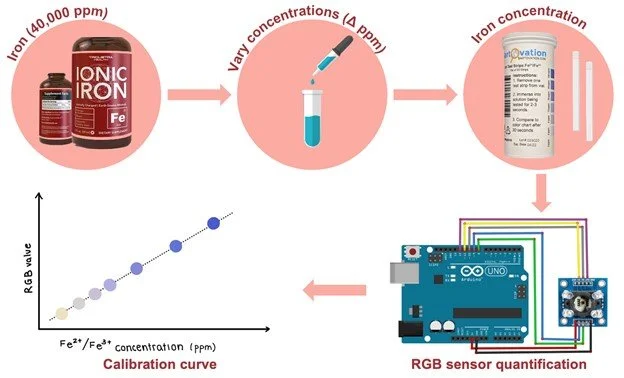
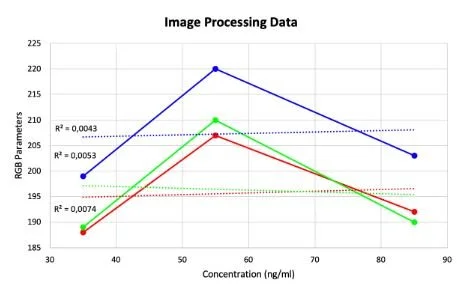
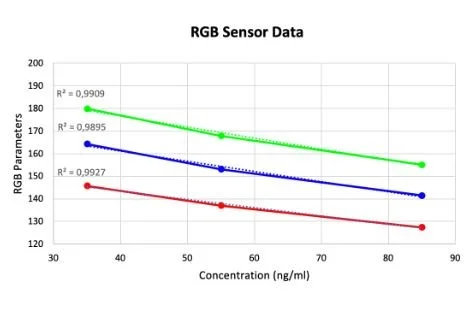
Here you can see data from our last set of experiments trying to quantify the result of our LFA tests for ferritin. For this experiment, we made solutions with known ferritin concentrations and applied them to the commercially available blood ferritin LFAs that we bought. The tests were then analyzed in our device to correlate the resultant test line saturation to specific ferritin concentrations. We attempted to quantify the LFAs and were able to generate this curve on the left from our RGB sensor data. The data looked promising as it forms a curve with an acceptable R squared value but unfortunately when we tried to verify the data using image processing we got conflicting results. One issue was commercially available and affordable LFA test strips for our biomarkers were hard to come by so we were only able to come up with three reliable data points. On top of this, we would need to engineer test strips for our biomarkers in order to ensure the resultant test line saturation is directly correlated to the concentration of our biomarker of interest. The image processing method was used as a comparison to our RGB results since that is another method of color quantification. Taking this into account our next steps in experimentation will be to engineer our own LFAs for our 4 biomarkers and try this test again and see if we can get an accurate correlation between resultant test line saturation and solution concentration with repeatable and reliable results.
Value Proposition
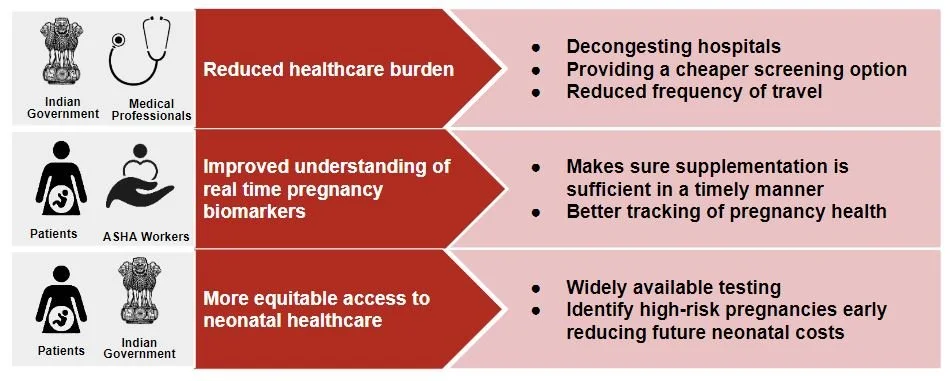
Our first value proposition directly benefits the healthcare system. Currently, women have to travel several kilometers to get a blood test, so the implementation of our device would allow reduced hospital visits and unnecessary repetitive tests.
The second value proposition benefits ASHA workers and patients. Monitoring these deficiencies enables ASHA workers and patients to have a better idea of what the next steps of pregnancy supplementation should be.
The third value proposition is more equitable access to neonatal healthcare. Nutrient monitoring can help identify which patients may be dealing with higher-risk births and address any concerns in a timely fashion and reduce future costs for the healthcare system.
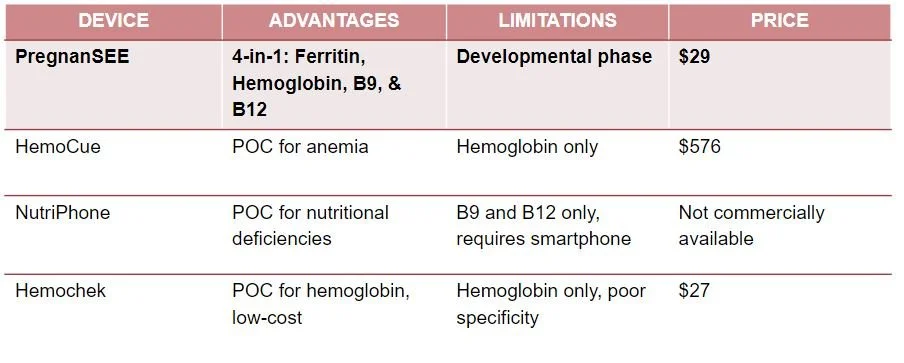
Competition
Here is a comparison of our product to point-of-care devices designed for nutritional monitoring that have been tested and implemented in rural India in some capacity. These work similarly to our device but none of these are in direct competition with our device as they only measure one to two of our 4 biomarkers. Through our stakeholder interviews, we found that qualitative hemoglobin checks such as Hemochek are used in rural India but this device only measures hemoglobin and is often inaccurate as the results contradict hospital lab tests.
Market Analysis
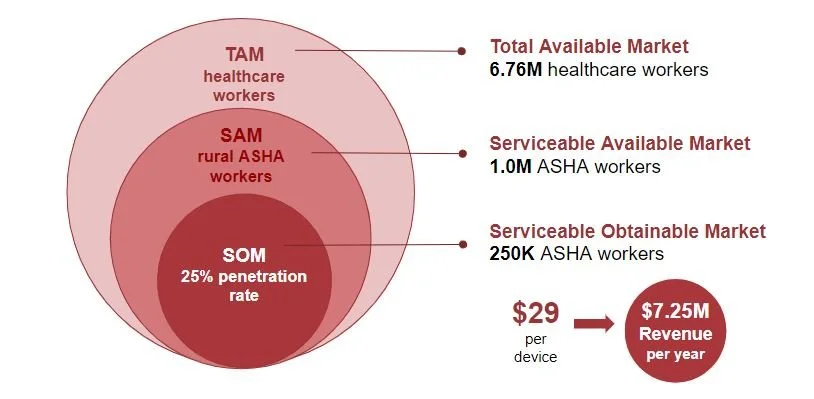
As for our initial market analysis, we decided to focus on healthcare workers who would be operating our device. We found that as of 2021 there are approximately 6.76 million healthcare workers in India which we choose as our total available market. Next, we define our serviceable available market as the 1M ASHA workers currently employed by the central government. Assuming a penetration rate of about 25% we get a serviceable obtainable market of 250K ASHA workers. Pricing our device at $29 we obtain a yearly revenue of 7.25 million dollars.
Business Model
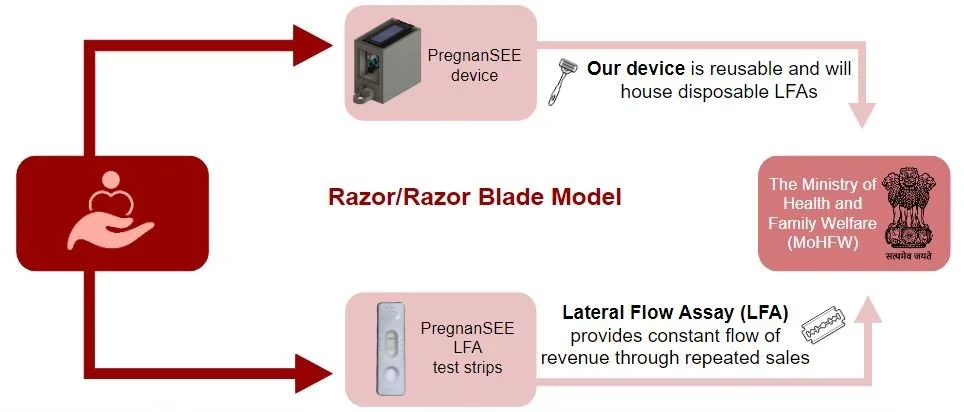
We intend to be a for-profit social enterprise and generate revenue through the razor/razor blade model. This would mean that the device is reusable but constant revenue is secured through the sale of a proprietary disposable component, the LFA. As we have mentioned, the existing healthcare infrastructure in India will accommodate our device through the ASHA workers. Regulatory mechanisms administered by the Ministry of Health and Family Welfare enable the funding of emerging technologies to provide ASHA workers with better tools to assess India’s rural pregnant population and provide accessible and effective quality healthcare.
Path to Market
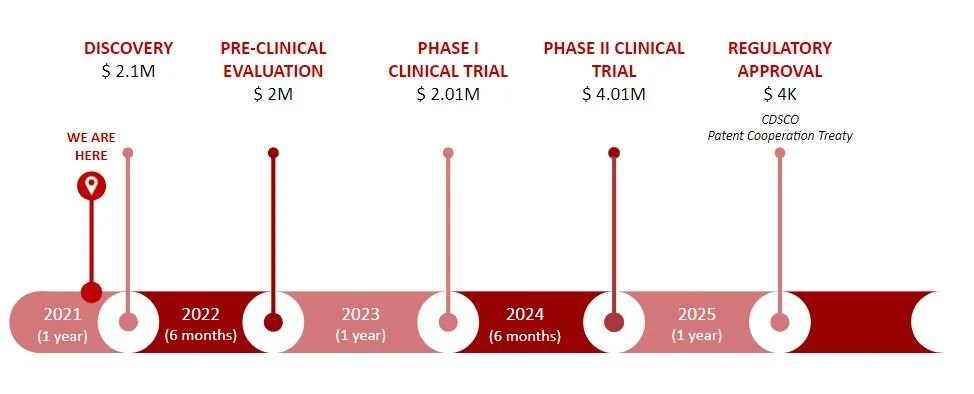
Our path-to-market is divided into five different stages. We are currently at the discovery stage, with a killer experiment performed and working towards our minimum viable product. We would then move to our pre-clinical evaluation to start with initial testing and get more data on the specifics of our device, such as safety and efficacy. For our clinical trials, we would assess ease of use for the ASHA workers and market implementation, starting in a few villages and then moving on to the states of Uttar Pradesh and Bihar to assess adoption by the rest of India. Finally, we would seek regulatory approval at the 4-year mark. To bring our product to market we would need $10M to help us navigate through these key milestones.
Future Directions
In the future, our total available market will consist of all countries with severe anemia prevalence in pregnant women, paying special attention to Southeast Asia and Africa. In addition, we would want to look into countries that already have an established rural healthcare infrastructure involving community health workers that would aid in the dissemination of our product as seen in Ghana and Afghanistan.
The Team
Our team consists of six graduate students at Columbia University, each equipped with diverse backgrounds and skill sets with a unified passion for global and women’s health. Additionally, multiple team members have direct ties to India providing access to key stakeholders.
Logo/ Mini Project
This was the culmination of a mini-project during our second semester that involved designing and building a physical logo for our team/device. The information about this project is on a separate page of this portfolio. You can click the button below to take you to that page!